Ondokuz Mayıs University Department of Chemistry
Special thanks to Assoc. Prof. Dr. Ahmet UYANIK.
Activated carbon
Among the most widely utilized industrial adsorbents, activated carbons are currently regarded as one of the most effective adsorbents in controlling environmental pollution. As a commercial product, activated carbons are obtained by activating carbons obtained from wood, peat, lignite, coal, charcoal, charcoal, bone, coconut shell, rice shell, hazelnut shell, and oil products through various processes.
In the early 1900s, patents were issued that formed the basis of current activated carbon production. These patents describe two basic principles of activated carbon production that are still valid today: chemical activation and gas activation. After 1920, activated charcoal was first used in water purification, but it was not widely used. However, since the unpleasant chlorophenol odor in drinking water caused a big problem in Germany in 1927, the use of activated carbon in the preparation of city water also gained great importance. Activated carbon was used in granular form at Hamm Water Works in 1929, and independently, in 1930 by Harrison in Bay City, Michigan, and in powder form by Spalding in 1929 to remove odors from drinking water. By 1932, 400 factories and by 1943, approximately 1,200 factories in America used activated carbon to control unpleasant odors.
General Properties of Activated Carbon
Activated carbon is a generic term used to describe the family of carbonaceous adsorbents with its large crystalline form and very large inner pore structure. Activated carbons are useful products that are harmless to human health and have a very high porosity and inner surface (5). Activated carbons are able to attract molecules and ions in solution through their pores to their inner surface and are therefore called adsorbents.
Surface Area
The inner surface of activated carbon (activated surface) is usually expressed as BET surface (m2/g). The surface area is measured using nitrogen (N2) gas. The inner surface area of activated carbon particles used in water treatment is desired to be approximately 1000 m2/g. Since the polluting substances will be retained on the surface of the activated carbon, the size of the surface area is a very effective factor in removing the pollutants. In principle, it is thought that the larger the surface area, the greater the number of adsorption centers. The numerical values related to the surface area and pore properties of activated carbon available in the literature are given below:
Numerical values related to surface area and pore properties of activated carbon.
Surface area: 400-1600 m2/g (BET N2)
Pore volume: >30 m3/100g
Pore width: 0,3 nm-1000 nm
The surface of the carbon particle attracts gaseous, liquid, and solid substances and forms a thin film layer on the surface, which means adsorption. There are two main reasons why activated carbon is preferred as an adsorbent. These are:
- It has an adsorbent surface to draw in certain substances,
- It has a large surface area to hold large amounts of substances
Pore Size
Another parameter that is effective in removing pollution is the pore size. Identifying the pore size is a very useful method for understanding the properties of carbon. The pores can be cylindrical or conical. The photo showing the pore structure of activated carbon below is taken with a Scanning Electron Microscope.
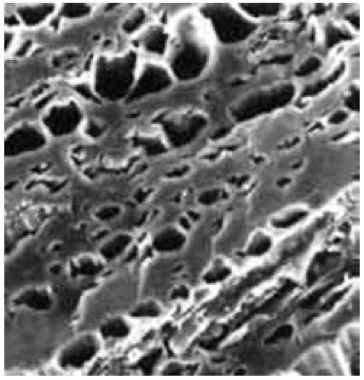
For adsorption, the pore structure is a more important parameter than the total inner surface. The size of the pores should be suitable for the particle diameters of the contaminants to be removed. Because the attraction force between the carbon and the adsorbed molecules is greater between molecules whose size is close to the pores.
Figure 1: Pore structure of activated carbon. Photograph taken with SEM
The International Union of Pure and Applied Chemistry (IUPAC) has divided the pore size of adsorbents into four groups according to their radius. That is:
- Macropores (r > 25 nm)
- Mesopores (1 < r < 25 nm)
- Micropores (0,4 < r < 1 nm)
- Sub-micropores (r < 0,4 nm) (Figure 2)
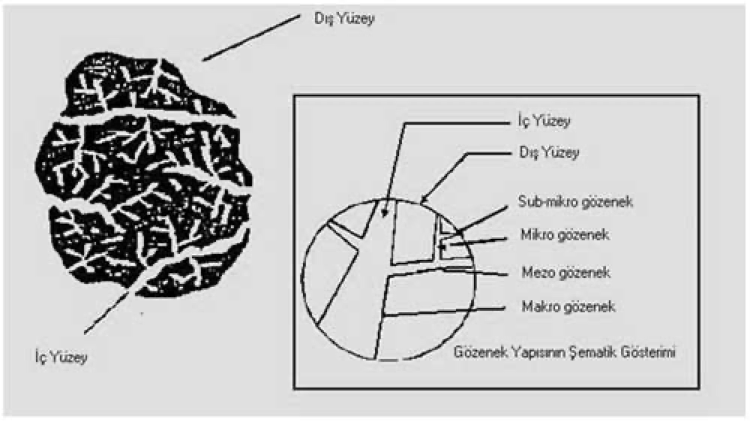
The activated carbon pore system, which is important for adsorption and desorption, is shown schematically in Figure 2. Micropores constitute a significant part of the inner surface (~95%).
Figure 2: Schematic diagram of activated carbon
Macropores are relatively unimportant for adsorption but they are necessary as conductors for rapid diffusion towards micropores (6). Macropores allow the molecules to penetrate into the activated carbon, mesopores allow it to be transported further into the inner parts, while micropores are used for adsorption.
Types of Activated Carbon
The best activated carbons used today for wastewater treatment are obtained from various coals and natural materials. These are coal, charcoal, peat, lignite, wood, bone; coconut, hazelnut, and rice shells; fruit seeds, and oil products. Activated carbons obtained from these materials are generally hard and dense. They can be used for a long time without decomposing in water. Activated carbons can be produced in different shapes with different properties. These are:
- Powder-activated carbon,
- Granular activated carbon,
- Pellet activated carbon, (also known as extruded activated carbon, columnar activated carbon).
As a result of the chemical activation of carbon, powder-activated carbons are obtained. These carbons are the most commonly used activated carbons in wastewater cleaning processes today. Granulated products and pellets made with gas activation are mostly used in the purification of gases. However, it is stated that granular activated carbons also give very good results in wastewater processing systems. Granular and powder-activated carbons give excellent results in the removal of organic and inorganic substances. These activated carbons have also been used for years to clean biologically treated wastewater and wastewater containing industrial effluents of organic origin.
Activation Techniques
For the production of activated carbon, all substances that are not carbon-poor can be used by activating them with various activation methods. These activation methods are classified into two categories: chemical activation and gas activation.
Chemical Activation
This technique is generally used for the activation of raw materials from peat and wood-based origin. The raw material is saturated with zinc chloride, phosphoric acid, or potassium hydroxide. It is then heated to 500-800 °C to activate the carbon. The activated carbon is washed, dried, and ground into powder. Activated carbons formed as a result of chemical activation are generally used for the adsorption of large molecules and exhibit a very large pore structure.
Gas Activation
This activation technique is generally used in the activation of coal and fruit peels. The raw material is first subjected to a thermal process called carbonization. This process helps to form a carbonaceous product with small pores. Then, the activation process is carried out in an inert gas atmosphere and at a temperature range of 800-1100°C. Thus, the intermediate material initially formed by carbonization is converted into the gas phase by the water-gas reaction given below, expanding the existing pores and increasing their number.
C + H20 ® CO + H2 -175,440 kJ/(kg mol)
This reaction is endothermic and the heat required for the reaction is maintained by the partial combustion of the CO and H2 formed.
2CO + O2 ® 2CO2 + 393,790 kJ/(kg mol)
2H2 + O2 ® 2H2O + 396,650 kJ/(kg mol)
The activated carbon obtained is classified and made ready for use by sieving and dust removal. Activated carbons obtained by gas activation also exhibit a good pore structure like those obtained by chemical activation. They are effectively used for the adsorption of molecules and ions from both liquid and gas phases.
Adsorption
Adsorption is defined as the accumulation and concentration of a substance on a surface or interface (7). The interface used in the definition can be the contact surface between a liquid and a gas, a solid, or another liquid. In other words, adsorption is the phenomenon of molecules sticking to the surface due to the forces of attacking the surface. It is stated that the adsorption of a dissolved compound by activated carbon occurs in three steps:
- Transport of adsorbed substance to the outer surface of the adsorbent,
- Diffusion of adsorbed material into the pores of the carbon, except for a small amount of adsorption occurring on the outer surface,
- Adsorption of solution on the inner surfaces of the adsorbent.
Another source states that adsorption occurs in three basic steps. These steps are:
- Film Diffusion: The solute molecules to be adsorbed enter the carbon particles and form a surface film.
- Pore Diffusion: Involves migration of solute molecules from the carbon pores to the adsorption center.
- Adhesion of Solute Molecules to Carbon Surfaces: Adherence occurs when the solute molecule binds to the carbon pore surface.
Adsorption Types
It is stated that adsorption on activated carbon can occur in three different processes (9).
Physical Adsorption
If adsorption occurs by unbalanced Van Der Waals forces on a surface, it is called physical adsorption. This type of adsorption is thermodynamically reversible. It is characterized by a low heat of adsorption and the degree of adsorption decreases as the temperature increases.
Chemical Adsorption
It is caused by the formation of a chemical bond with the monomolecular layer of the adsorbed substance on the surface due to the valence forces of the surface molecules. Adsorption requires high temperature and is not thermodynamically reversible. If the temperature is too high, the physical adsorption event can turn into chemical adsorption (10).
Electrostatic Adsorption
It is defined as the effect caused by the forces of electrical attraction responsible for the adsorption of solutions onto activated carbon. Also, the electrical attraction between the negatively charged carbon particles and the positively charged adsorptive molecules or ions reduces the barriers that appear during diffusion and thus increases the adsorption efficiency.
Regeneration of Adsorbents
As molecules are adsorbed onto the adsorbent surface, less space is left for new molecules to adsorb and as a result, the adsorbent loses its adsorption efficiency. The process of restoring its ability of adsorption efficiency to the adsorbent is called ‘‘recovery’’. The physical strength of the activated carbon must be large enough to withstand the entire recovery process. However, over time, a small amount of activated carbon is lost or oxidized due to thermal expansion, shrinkage, and finally the breakdown of the structure.
Adsorption and Desorption on a Solid Surface
Adsorption, among other definitions, is the enrichment of solutes by adsorption on the attached surface of a solid adsorbent. The bonding forces between the atoms on the surface of the adsorbent called the active center, are not fully saturated. Adsorption of foreign molecules takes place on these active centers. A substance adsorbed on an adsorbent is displaced by a substance adsorbed more strongly than itself. The displaced substance is desorbed or released by the carbon. This phenomenon continues throughout the adsorption of more preferred types. Chemical adsorption occurs when the functional groups of the adsorbed substance and the adsorbent interact to form a stable bond. Desorption is more suitable for physically adsorbed substances rather than chemically adsorbed substances. The adsorption and desorption processes on the solid phase are shown schematically in Figure 3.
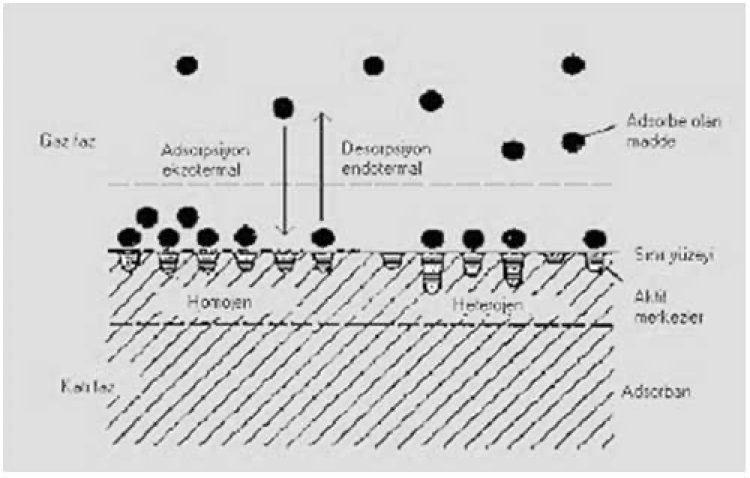
Figure 3: Adsorption and desorption on a solid surface
